Dimethyl ether (DME) Fact Sheet
Introduction and basic data on DME
State of the Art for DME production
Major stakeholders in DME in the EU
Introduction
Dimethyl ether (typically abbreviated as DME), also known as methoxymethane, wood ether, dimethyl oxide or methyl ether, is the simplest ether. It is a colourless, slightly narcotic, non-toxic, highly flammable gas at ambient conditions, but can be handled as a liquid when lightly pressurized. The properties of DME are similar to those of Liquefied Petroleum Gas (LPG). DME is degradable in the atmosphere and is not a greenhouse gas.
Molecular Formula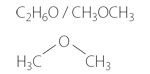
Comparison of Fuel Properties
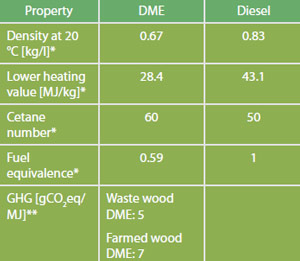
Source: *FNR 2012. Median values are used for simplification. Please refer to standards for ranges. **Directive 2009/28/EC, total for cultivation, processing, transport and distribution.
Utilization
Substitute for diesel fuel; transportation fuel; power generation fuel; domestic gas
Relevant fuel regulations
EN590 (diesel fuel)
Main feedstocks
Forest products, agricultural by-products, organic waste, energy crops, black liquor
Scale of Production
Demonstration scale
Production process
DME is primarily produced by converting natural gas, organic waste or biomass to synthesis gas (syngas). The syngas is then converted into DME via a two-step synthesis, first to methanol in the presence of catalyst (usually copper-based), and then by subsequent methanol dehydration in the presence of a different catalyst (for example, silica-alumina) into DME.
The following reactions occur:
2H2+ CO ![]() CH3OH
CH3OH
2CH3OH ![]() CH3OCH3 + H2O
CH3OCH3 + H2O
CO+H2O ![]() CO2+H2
CO2+H2
Alternatively, DME can be produced through direct synthesis using a dual-catalyst system which permits both methanol synthesis and dehydration in the same process unit, with no intermediate methanol separation, a procedure that, by eliminating the intermediate methanol synthesis stage, the licensors claim promises efficiency advantages and cost benefits.
Both the one-step and two-step processes are commercially available.
DME can also be converted itself into olefins and synthetic hydrocarbons.
State of the Art
The DME demonstration plant in Piteå, Sweden, which was put into operation in 2010, is the only gasification plant worldwide producing high-quality synthesis gas based on 100% renewable feedstocks. The raw material used is black liquor, a high-energy residual product of chemical paper and pulp manufacture which is usually burnt to recover the spent sulphur.

Applications
Due to its good ignition quality, with a high cetane number, DME can be used in diesel engines as a substitute for conventional diesel fuel. However, compared to diesel fuel DME has a lower viscosity (insufficient), and poor lubricity. Like LPG for gasoline engines, DME is stored in the liquid state under relatively low pressure of 0.5 MPa. This helps to limit the number of modifications required to the engine. Still, some slight engine modifications are necessary, primarily relating to the injection pump and the installation of a pressure tank, similar to that for LPG. The fuel line must also be adapted with specific elastomers.
DME in diesel engine burns very cleanly with no soot.
The infrastructure of LPG can be used for DME. As part of the FP7 project BioDME, under the leadership of the Volvo Group, DME production is being optimized, especially for use as a transport fuel.
EC-funded projects on DME
See R&D Funding page for further project details
BioDME - Production of DME from Biomass and utilisation as fuel for transport and for industrial use. Funded by 7th Framework Programme and Swedish Energy Agency.
Major stakeholders
Some major DME stakeholders in the EU are listed below:
Volvo Group, Sweden
Chemrec,, Sweden
Haldor Denmark
Preem, Sweden
Total, France
Further Information
See the BioDME page for updated project information
